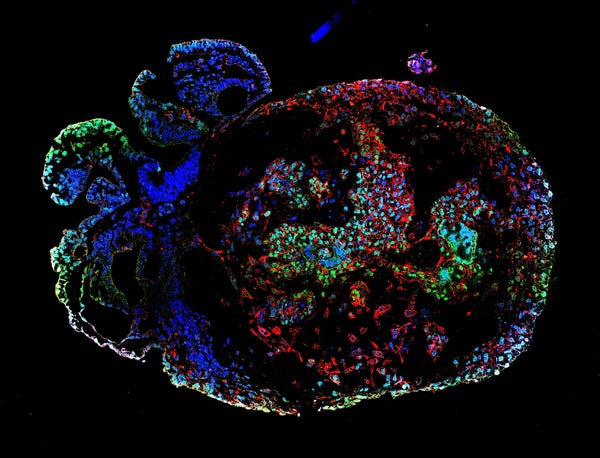
Scientists have cultivated monkey embryos in the laboratory long enough to watch the beginning of organ formation and the development of the nervous system — milestones that are difficult to observe in embryos growing in the uterus. The embryos reached the age of 25 days, making them what might be the oldest primate embryos to be grown outside the womb.
Independent teams described the findings in separate papers in Cell on 11 May.
“It’s very impressive,” says Magdalena Zernicka-Goetz, a developmental biologist at the California Institute of Technology in Pasadena, who was not involved in the research. “It’s going to bring a lot of new insights.”
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Going 3D
Few things are as difficult as keeping lab-grown embryos alive for longer than a couple of weeks — most amount to nothing more than a mixed bag of cells in a dish. Previously, both teams had managed to culture monkey blastocysts — balls of dividing cells — in Petri dishes for up to 20 days. Past that point, all the embryos had collapsed, making it impossible to see more advanced stages of their development, such as early signs of the nervous system and organ formation.
A 25-day-old monkey embryo stained with conventional dye (pink). Credit: Zhai et al./Cell
But in the new studies, the researchers grew monkey embryos in small vials of culture medium, which allowed the embryos to grow in three dimensions as they would inside the womb. Both teams coaxed their embryos to survive for 25 days after fertilization. Neither the authors nor outside scientists contacted by Nature knew of older primate embryos grown in the lab.
Watching organs take shape
Hongmei Wang, a developmental biologist at the State Key Laboratory of Stem Cell and Reproductive Biology at the Chinese Academy of Sciences in Beijing, and her team obtained egg cells from female cynomolgus monkeys (Macaca fascicularis) and fertilized them in the lab with sperm collected from their male counterparts. A week later, they placed the resulting blastocysts into a gel-like substance in small cylindrical containers and watched them grow for 25 days.
Roughly two weeks after fertilization, more than half of the embryos had an embryonic disk — a flat mass of cells. These disks eventually formed the three main cell layers of the body: the endoderm, mesoderm and ectoderm. The lab-grown embryos also showed genetic features similar to those seen in natural monkey embryos within the same time frame.
By day 20, the embryos had developed a neural plate — one of the early hallmarks of the nervous system. As in natural embryos, this plate thickened and bent into a tube that forms the basis of the brain and spine. Wang and her team also pinpointed cells that would eventually become motor neurons. The insights gleaned from the lab-grown embryos will help researchers to develop a better understanding of early embryo development in primates, says Wang.
Where blood is born
In the second study, Tao Tan, a developmental biologist at Kunming University of Science and Technology in Yunnan, China, and his colleagues also generated blastocysts from cynomolgus monkey eggs and sperm. But they used two different types of cell culture to provide stronger mechanical support for the embryos, and added glucose to provide them with energy as they grew.
As in Wang’s study, most of the cells in the cultured monkey embryos were the same type as those typically seen in natural embryos 18 to 25 days after fertilization. When Tan and colleagues took a closer look at the embryos’ mesoderm cells, they found that some had differentiated into heart muscle cells and others had matured into cells found in the lining of blood and lymphatic vessels. The team also pinpointed cells that develop into connective tissue and ones that form the foundation of the digestive system.
The researchers also found signs that blood cells and their components were beginning to take shape in the yolk sac, which supplies embryos with nutrients. “We were deeply impressed,” says Tan. These blood cells “are almost impossible to obtain during human embryonic development.”
Naomi Moris, a developmental biologist at the University of Cambridge, UK, says that the studies present an important step in developing methods that can sustain embryos outside the womb for longer than had been possible previously. But she cautions that there is still a long way to go before lab-grown embryos will look and behave like the real thing. “They still look a bit different to how we would expect [them] to look at these stages,” says Moris, who was not involved in the research. “There’s definitely still scope for improvement.”
This article is reproduced with permission and was first published on May 11, 2023.