Kimberly Chauche, a corporate secretary in Lincoln, Neb., says she’s always been overweight. When she was as young as five years old, her doctors started trying to figure out why. Since then her life has involved nutritionists and personal trainers, and eventually she sought therapists to treat her compulsive eating and weight-related anxiety. Yet answers never arrived, and solutions never lasted.
At 43, Chauche was prescribed a weight-loss medication called Wegovy—one of a new class of drugs that mimic a hormone responsible for insulin production. She took her first dose in March 2024, injecting it into herself with a needle. Within a couple of months she had lost almost 20 pounds, and that felt great. But the weight loss seemed like a bonus compared with a startling change in how she reacted to food.
She noticed the shift almost immediately: One day her son was eating popcorn, a snack she could never resist, and she walked right past the bowl. “All of a sudden it was like some part of my brain that was always there just went quiet,” she says. Her eating habits improved, and her anxiety eased. “It felt almost surreal to put an injector against my leg and have happen in 48 hours what decades of intervention could not accomplish,” she says. “If I had lost almost no weight, just to have my brain working the way it’s working, I would stay on this medication forever.”
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Chauche is hardly alone in her effusive descriptions of how Wegovy vanquished her intrusive thoughts about food—an experience increasingly referred to as the “quieting of food noise.” Researchers—some of whom ushered in the development of these blockbuster drugs—want to understand why.
Among them is biochemist Svetlana Mojsov of the Rockefeller University, who has spent about 50 years investigating gut hormones that could be key to regulating blood glucose levels. In seeking potential treatments for type 2 diabetes, Mojsov ultimately focused on one hormone: glucagonlike peptide 1, or GLP-1. Her sequence of the protein in the 1980s became the initial template for drugs like Wegovy. The medications, called GLP-1 receptor agonists, use a synthetic version of the natural substance to activate the hormone’s receptors. The first ones arrived in 2005. In 2017 the U.S. Food and Drug Administration approved semaglutide—now widely known as Ozempic.
In the years since Ozempic came to market, GLP-1 drugs have catapulted to stardom, becoming a multibillion-dollar industry with a surreal series of successes, first as an effective treatment for diabetes and then as a hit for weight loss. Wegovy, a version of semaglutide specifically for weight loss, became available in 2021. Both drugs were created by Novo Nordisk; other pharmaceutical companies have developed similar ones. One 2024 survey in the U.S. found that one in eight adults reported having taken a GLP-1 drug.
Mojsov and other researchers know that these drugs make people lose weight because they reduce appetite and thus food intake. They make people feel fuller, faster. But scientists don’t have a technical definition for so-called food noise, and they are only beginning to understand how synthetic GLP-1 acts not just in the digestive system but in the brain. This work is illuminating neurobiological explanations for hunger and satiety, pleasure and reward—as well as why these sensations so critical to survival might get dysregulated, causing compulsive behaviors and addictive patterns. “That’s what we need to understand now,” Mojsov says. “The next frontier is to understand the biology behind the Ozempic effects on the brain.”
GLP-1 is one of many essential gut hormones that help to control eating behavior, nutritional absorption, digestion, and the overall balance of energy coming into and being used by the body. Over the past few decades several hormones from various body systems involved in food intake have been targeted as potential treatments for obesity and diabetes, but “GLP-1 seems to be the one that’s risen to the top, at least for pharmacotherapy,” says Scott Kanoski, a behavioral neuroscientist and professor at the University of Southern California.
That’s in part because it belongs to a batch of hormones called incretins, which prompt insulin production in response to food ingestion. In a 1987 study, Mojsov and her collaborators injected GLP-1 into a rat pancreas model to see whether it stimulated insulin secretion. “It was a beautifully clear-cut result,” Mojsov says. “As the GLP-1 levels went up, insulin levels went up.”
Now Medical Studios
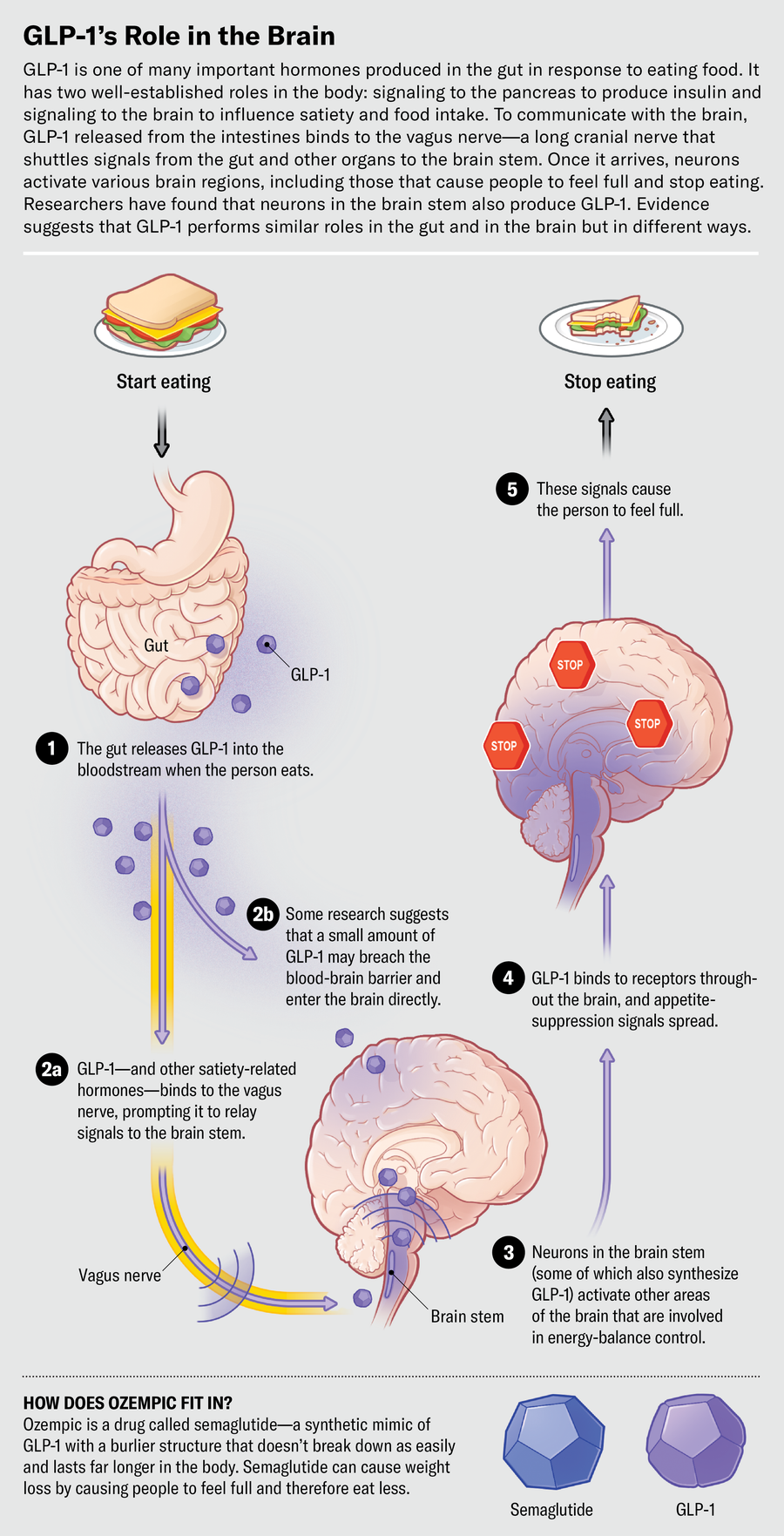
The basic pathway of GLP-1 in the gut goes like this: When a person eats a meal, a cascade of hormones, including GLP-1, is released to aid food absorption and digestion. As the food gets broken down into glucose and other molecules in the digestive tract, GLP-1 gets released from the intestine. Levels of the hormone rise slowly, then spike to signal fullness.
Some GLP-1 in circulating blood binds directly to receptors in the pancreas to prompt the release of insulin. The hormone also can latch on to receptors on the vagus nerve—a long cranial nerve that shuttles messages between the brain and organs throughout the body. While a person eats, hormonal messages traveling via the vagus nerve tell their pancreas to produce insulin, which converts glucose to energy and brings blood glucose levels back down. The rise and fall of blood sugar can influence hunger and satiety.
GLP-1 is short-lived in its natural form. Within one or two minutes the molecule gets dismantled by enzymes in the blood and cleared by the kidneys. So in the 1990s drug companies began creating synthetic versions of GLP-1, hoping to land on a durable, longer-lasting structure. Scientists found success in a compound in Gila monster saliva that is similar to human GLP-1 but much more stable. They attached a long chain of lipids that can bind to albumin—a protein in blood that serves as a carrier for the drug—and keep the compound active for hours or even days.
Around 2021 the story of GLP-1 receptor agonists took a dramatic turn. Demand for the drugs soared as celebrities and social media influencers began sharing their experiences using Ozempic off-label to achieve incredible weight loss. As more and more people took the drugs, stories spread about “food noise,” and researchers started looking even more closely at what was going on in the brain.
Matthew Hayes, a nutritional neuroscientist at the University of Pennsylvania, who has studied GLP-1 and other gut hormones since 2006, explains that these drugs work partly because they slow digestion and modulate glucose levels. Some metabolic effect is causing weight loss, but it’s “only contributing to a small degree,” he says. “The way in which GLP-1 drugs are causing weight loss is without question due to suppression of food intake—to satiety,” Hayes adds. Increased satiety means people eat smaller, less frequent meals.
Scientists have known for some time that GLP-1 seems to have a secondary function as a satiety signal—a ping to the brain to stop eating. In 1996 researchers injected GLP-1 directly into the brains of hungry rats, and the rodents’ food intake decreased by as much as 95 percent. The study was some of the first evidence that the hormone had an effect in the brain. “All these feelings we have about being hungry or full are fundamentally brain-driven,” explains Herman Pontzer, an evolutionary anthropologist at Duke University and author of Burn, a book about the science of metabolism. “It makes sense that that’s where the mechanism of action is.” The brain was always involved, he says, but these new drugs are helping researchers zero in on the brain as a “center for regulating energy in and energy out.”
Appetite—the drive to eat—is biologically motivated by three core sensations: hunger, fullness and reward. “All three speak to each other, and for that, parts of the brain play a role,” explains Giles Yeo, a University of Cambridge professor who specializes in the genetics of body weight and the neuroscience of food intake. The hypothalamus—an almond-shaped structure near the base of the brain—is involved with feelings of hunger or starvation; the hindbrain, including regions of the brain stem, plays a role in fullness; and a distributed network fanning out from the midbrain to the prefrontal cortex orchestrates reward elements. It produces “the nice feeling you feel from eating chocolate that you don’t get from eating broccoli,” Yeo explains.
These brain regions all sense signals communicated via the gut-brain axis network—and scientists have found they are studded with GLP-1 receptors. “The receptor-expressing cells are everywhere, everywhere, throughout the brain,” Hayes says. “It’s almost a question of where are they not?” In fact, these receptors are now known to be abundant throughout the body. He thinks the reason so many cells and neurons are making GLP-1 receptors must be “because they want to respond to it.”
When GLP-1 released from the gut latches on to the vagus nerve, the nerve sends signals up the brain stem to the nucleus tractus solitarius (NTS), a bundle of sensory neurons deep in the brain. The NTS is “the first place that [receives] all incoming satiety signaling from the gut,” Hayes says. “It’s like a processing hub for energy-balance control.”
Because of its short lifespan, it’s unlikely that natural GLP-1 produced in the intestines reaches high enough concentrations in the brain to affect satiety. But the NTS doesn’t just relay incoming satiety signals from the gut—it also produces GLP-1 itself. Although the details of the mechanism are not yet fully understood, researchers have found that the primary source of GLP-1 in the brain is preproglucagon (PPG) neurons in the NTS. When activated, they act like an emergency brake at the end of the meal, flooding the brain with GLP-1 to send the message to stop eating. This effectively shuts down areas in the brain involved in feeding response, homeostatic controls, energy balance and decision-making about food—as well as the liking and wanting of food and impulsive behaviors associated with eating. For people with obesity, these neurons and hormonal activity might be a clue—one that the new drugs are bringing to light.
Compared with the naturally occurring hormone, the drugs have a stronger structure that better withstands degradation and allows them to be bioactive for hours—the newest formulas can last up to a week. This gives them the potential to act on the brain and stimulate those receptors for longer periods, Mojsov says.
There’s growing evidence in animal models that the drugs make it through the blood-brain barrier—a protective membrane surrounding most of the organ— by penetrating “leaky” areas, such as the NTS. One way they get in is by riding on tanycytes, cells that aid in communicating energy balance between the peripheral and central nervous systems and enable nutrients, hormones and drugs to cross the blood-brain barrier.
“What’s interesting with these GLP-1-based drugs is that they’re lasting a lot longer than [natural] GLP-1,” Kanoski says. “This opens up a whole new pathway for communication.” Hayes says researchers are now looking into how much GLP-1 gets in, where exactly the drugs go, and what behaviors or functions they cause. “How deep into the brain do they get?” Hayes asks.
“My whole life was thinking about food,” says Meranda Hall, a 32-year-old administrator at a law firm in New York City. Hall was a cross-country runner in high school, and she kept exercising daily into adulthood. But she ate almost constantly and had been carrying extra weight since childhood. Even when Hall felt physically full, her brain was occupied by thoughts of food. “While I was eating,” she says, “I’d be thinking about the next meal.”
In August 2023, when Hall began taking Wegovy, she weighed 271 pounds. Nine months later she’d lost 78 pounds—as well as her intrusive thoughts about eating. The vanishing compulsion to overindulge didn’t stop with food, though. Hall says she used to be an enthusiastic social drinker, “an eight-margs-at-Taco-Tuesday type of girl.” Now she’s “a sober Sally.”
Like Hall, some people using GLP-1 receptor agonists report not only a decreased desire for food but reduced cravings for alcohol, nicotine, drugs, online shopping, nail picking—the list goes on. These effects are driving a spate of research into possible overlapping circuitry linking compulsive behaviors, appetite and satiety.
Neurons that produce dopamine—a chemical with pivotal involvement in motivation and pleasure—project to the nucleus accumbens, a midbrain structure important for experiencing reward, explains Patricia Sue Grigson, a neuroscientist and addiction researcher at Penn State College of Medicine. Like other brain structures, the nucleus accumbens has GLP-1 receptors. Studies have shown that in animals, dopamine release peaks after they eat a sweet meal of sucrose—and after they are exposed to cocaine or opioids. “But when there’s a GLP-1 agonist onboard, that’s pretty much squelched,” Grigson says. “You don’t get a peak to those rewards.”
In human experiments, scientists have observed that the same neurological pathways are stimulated when people gamble or take cocaine—or when their blood sugar levels have been artificially changed to stimulate fasting. Janice Jin Hwang, chief of the division of endocrinology and metabolism at the University of North Carolina at Chapel Hill, explains that “there are these networks of brain regions that have been very well characterized, mainly in addiction literature, that control our desire and motivation for food but also for addicting things.”
One reason food lights up reward pathways is that it’s essential for survival, says Lorenzo Leggio, an addiction researcher at the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. Visuals, taste, smell, memory, and other cues work together to reinforce food-seeking behavior, and “GLP-1 is trying to keep that process somehow under control,” Leggio says. “You start eating your cake. You love it, but what is preventing you from eating, like, 20 pieces of cake? GLP-1 is one of the triggers that is increasing your satiety,” he explains. “It’s reducing your reward, your pleasure, for that cake.”
Grigson and Leggio are among a growing number of researchers studying the effects of GLP-1 medications on this reward pathway for potential addiction treatments. In a recently completed clinical trial, Grigson tested the safety and efficacy of daily injections of a GLP-1 receptor agonist, liraglutide, in people receiving treatment for opioid use disorder. They saw an approximately 40 percent reduction in opioid cravings. (Results are not yet published.)
Grigson’s team found that administering GLP-1 drugs in combination with buprenorphine, a current treatment for opioid use disorder, was also highly effective. Buprenorphine is an opioid itself, and people taking it as a medication may continue to experience drug cravings. Grigson hopes that adding GLP-1 medicines might help reduce the amount of buprenorphine needed. She is currently conducting a multisite follow-up study with researchers at New York University to investigate the treatment’s effects on withdrawal. In May, Novo Nordisk announced that an upcoming clinical trial would investigate the drug as a treatment for liver disease—and explore its effects on alcohol consumption.
Endocrinologist Ania Jastreboff tells her patients that GLP-1 medications may change their desire to eat. But not everyone experiences the same dramatic effects. “I kind of preface by saying we don’t know who will respond and how they will respond, how much weight a certain individual may lose, and how that may also impact their health overall,” says Jastreboff, who is the director of Yale University’s Obesity Research Center. Some people on semaglutide have lost as much as 20 percent of their body weight. But in one study, nearly 18 percent of users lost less than 5 percent. Some people are unable to tolerate the drugs because of their side effects, particularly severe nausea and diarrhea—a 2021 study showed 4.5 percent of people taking semaglutide discontinued the drug because of gastrointestinal issues. Scientists are now seeking to understand why the efficacy seems to vary so dramatically.
Natural GLP-1 levels may differ from person to person, and that could possibly explain varying susceptibility to weight gain or diabetes. Yeo studies why some people eat too much and says maybe it’s because they “feel less full for every given mouthful of food they eat. And part of that could be because their GLP-1 levels don’t go up as high for a given meal.” In those people, Yeo says, synthetic GLP-1 drugs may work better than they would in people with naturally higher levels.
Hayes wonders whether people who aren’t responsive to the drugs might have mutations in their GLP-1 receptors—and whether that could be part of why they gained excess weight in the first place. He speculates that GLP-1 receptors in some people may have genetic differences that might influence how well the hormone binds to the receptor and activates subsequent insulin and satiety pathways.
Pharmaceutical companies are now creating even more potent weight-loss medications by targeting multiple gut hormone receptors at once. Eli Lilly’s tirzepatide uses synthetic versions of GLP-1 and gastric inhibitory polypeptide; clinical trials revealed it caused people to lose more than 25 percent of their body weight over 88 weeks.
The U.S. clinical trial registry shows that thousands of studies on GLP-1 receptor agonists are underway now. A large, multiyear study that showed semaglutide reduced risk of heart attack and stroke by 20 percent helped Wegovy gain FDA approval as a treatment for cardiovascular disease earlier this year. Weight reduction most likely played a large role in heart health, but researchers are also finding convincing early evidence that GLP-1—and the drugs—may reduce inflammation when bound to receptors. That observation is now opening up the drugs to clinical trials for diseases that seem less obviously related to metabolic disorders, including Alzheimer’s, Parkinson’s, depression and even cancer.
As new findings emerge, GLP-1 medications are changing how researchers and clinicians think about body weight. Health issues that manifest as diabetes or obesity have been primarily considered peripheral disorders—problems of the pancreas, liver or body tissue—Hwang says, but this is only part of the picture. Jastreboff, who has been working on obesity treatments for 15 years, says the drugs are probes to better understand the physiology of obesity. “They’ve enabled us to have a conversation about obesity as a complex neurometabolic disease,” she says.
For so long people who couldn’t lose weight and keep it off have been told that their willpower simply wasn’t strong enough, says Daniel Drucker, an endocrinologist at the University of Toronto, who researched GLP-1 alongside Mojsov in the 1990s. “We—including health-care professionals—would blame people challenged by their inability to lose weight,” he says. “It’s hard to think of diseases where we blame the individual. You would never say, ‘Your cancer came back; you didn’t really try hard enough.’” The study of GLP-1 could help erode the stigma associated with obesity and addiction by replacing assumptions with clear pathology.
“We all have the same reward systems that are absolutely essential to normal functioning,” Pontzer says, “and it’s only when we get toward the real far end of the spectrum on those reward responses that we get into trouble.” This hormonal system is evolutionarily ancient. “And we are now, in 2024, finding the advantages of the system through these drugs—we have hijacked it, if you will,” Hayes says. “We are at the precipice of the beginning.”

